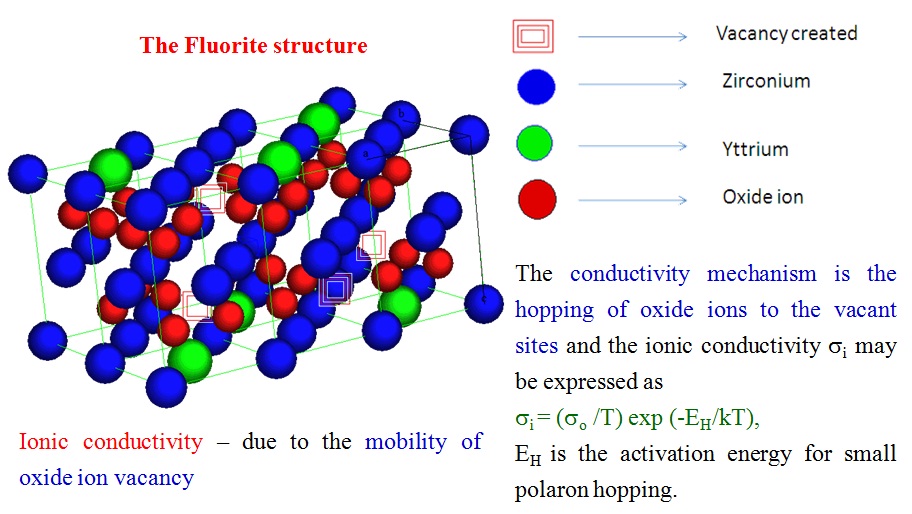
IONIC CONDUCTIVITY
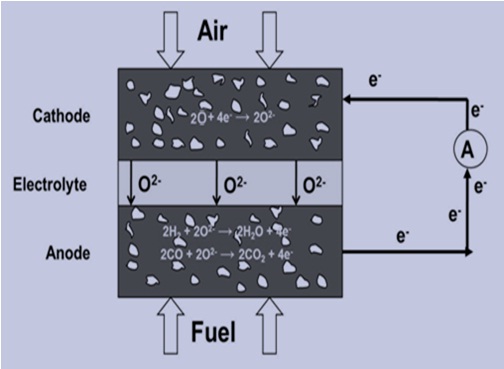
WORKING OF SOFC
Air flows along the cathode, when an oxygen molecule contacts the cathode/electrolyte interface; it acquires four electrons from the cathode and splits into two oxygen ions.Air flows along the cathode, when an oxygen molecule contacts the cathode/electrolyte interface; it acquires four electrons from the cathode and splits into two oxygen ions.
These oxygen ions diffuse into the electrolyte material and migrate to the other side of the cell where they encounter the anode/electrolyte interface.
Here they react, giving off water, carbon dioxide and heat. The electrons go through the anode to the external circuit and return to the cathode , thereby producing electrical electrochemical device that converts the chemical energy in oxygen and hydrogen to react.Here they react, giving off water, carbon dioxide and heat. The electrons go through the anode to the external circuit and return to the cathode , thereby producing electrical electrochemical device that converts the chemical energy in oxygen and hydrogen to react.
Crystal structure of 8 mol %Yttria stabilized Zirconia (electrolyte material)
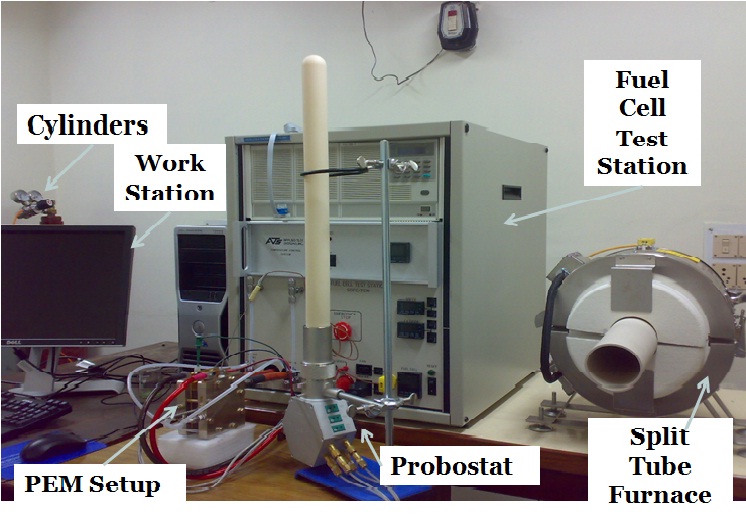
Fuel Cell Test Station Setup
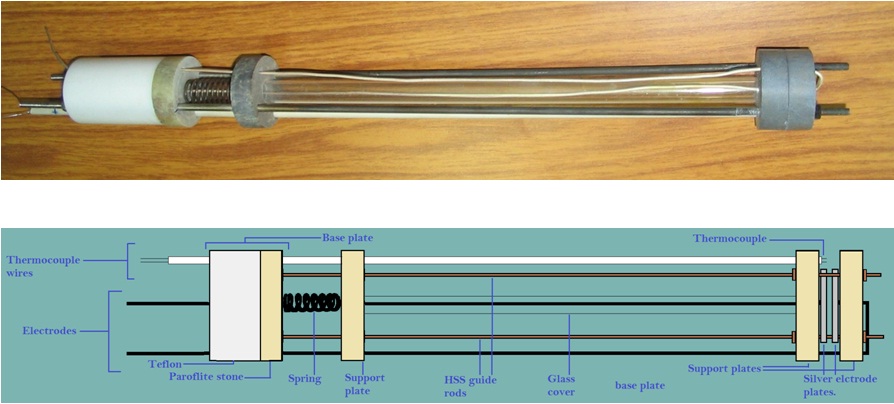
Schematic of sample holder used for ionic conductivity measurements